What is manufacturing operations management?
Before we dive into the five reasons to adopt a manufacturing operations management system, let’s discuss what MOM is. Manufacturing operations management is a holistic solution that provides full visibility into manufacturing processes so that manufacturers can steadily improve manufacturing operations performance. As part of the evolution of a manufacturing execution system (MES), a MOM system consolidates all production processes to improve quality management, advanced planning and scheduling, R&D management and more.
Digitalizing these manufacturing areas can further optimize production performance to improve efficiency, flexibility and time-to-market. Medical device manufacturers with fully digitalized processes are better equipped to respond to market changes and provide innovation rapidly.
The role of MOM in the medical device industry
Manufacturing operations management creates a digital foundation for enabling enforceable, proactive and paperless manufacturing. It identifies, analyzes and prevents errors, integrating advanced planning and scheduling as well as manufacturing intelligence capabilities. For the medical device industry, MOM creates a self-auditing electronic device history record (eDHR)/electronic batch record (eBR) to support regulatory compliance using a digital representation of the manufacturing processes – the digital twin.
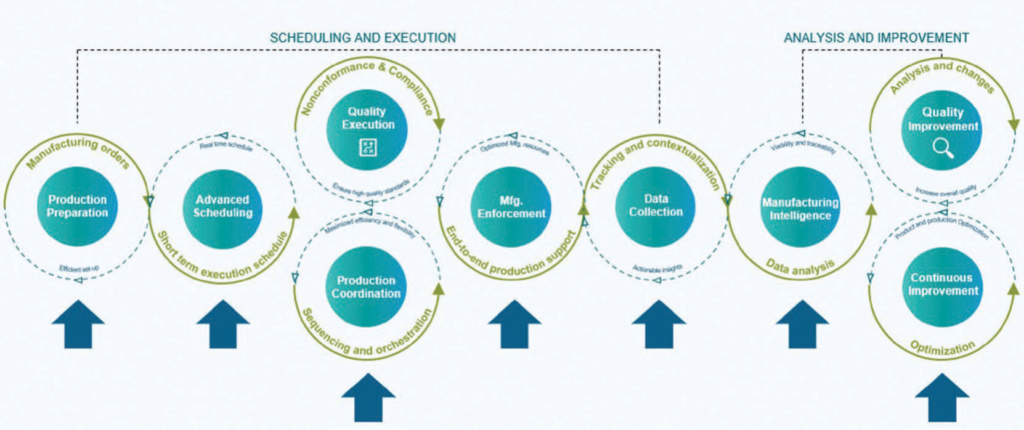
The manufacturing operations management workflow can be connected to the larger manufacturing value chain. This provides systemic feedback to design as well as enterprise planning. This creates closed-loop manufacturing, which is critical to supporting innovation.
Five reasons why manufacturers need MOM
- Orchestration – MOM provides the orchestration and planning of manufacturing and quality operations.
- Vertical integration – MOM bridges the gap between enterprise systems and shop floor automation.
- eDHR/eBR – MOM automatically generates the eDHR/eBR, supporting more rapid compliance processes.
- Digital twin – MOM implements the comprehensive digital twin of the physical production realm, creating a digital representation of the manufacturing process.
- Analytics – MOM transforms big data into IoT actionable information (smart data) and provides continuous improvement and innovation intelligence.
To learn more about trends in the medical device industry, unique challenges that manufacturers face and requirements for digital infrastructure, download the free ebook here.


